Brain-computer interface (BCI) developer Paradromics announced today that the FDA accepted it into its Total Product Life Cycle Advisory Program (TAP). TAP provides early and frequent strategic engagement from the FDA, patients, providers and payers. It facilitates rapid development and widespread access to medical devices. Paradromics can benefit from more timely premarket interactions, earlier identification and mitigation of device development risk and a more efficient premarket review for its Connexus system.
Related: FDA issues 510(k) approval for Epica’s See Factor CT3 system
Paradromics already has two FDA breakthrough device designations for its Connexus system. One recognizes its potential to help patients communicate after losing the ability to speak. The second covers the severe loss of movement to control computer devices.
Connexus enables communication for people with conditions like ALS, spinal cord injury and stroke. These conditions may cause severe motor impairment, affecting the patients’ ability to communicate. Future applications could include treatment-resistant mental health conditions, like depression.
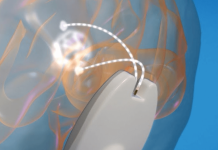
The fully implantable BCI can record from single neurons, using durable materials and packaging. According to a news release, advantages include the ability to obtain high-resolution data over long periods of time. This data can enable complex applications like decoding intended speech.
In preparation for a clinical trial in 2025, the company also launched a patient registry so patients can submit interest.
“We are happy to partner more closely with the FDA,” said Paradromics CEO Matt Angle. “Building a device that senses neural signals with single neuron resolution and works reliably in the body for years was challenging. There are easier approaches, but they aren’t as good for patients. We want to deliver the best possible device on the safest possible timeline, and so we appreciate access to the TAP program.”