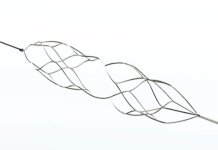
Emergency Scientific announced the first patient use of its Landmark resuscitative endovascular occlusion of the aorta (REBOA) catheter.
Salt Lake City-based Emergency Scientific developed Landmark for REBOA, a procedure developed by the military. It aims to treat hemorrhage from non-compressible fractures of the pelvis and penetrating trauma of the abdomen. Civilian providers since adopted the procedure as well.
Related: Ohh-Med launches upgraded version of erectile dysfunction wearable
REBOA also treats postpartum hemorrhage (PPH), cardiac arrest (CA), abdominal aortic aneurysm (AAA) ruptures and gastrointestinal (GI) bleeds.
Dr. Scott T. Youngquist, an emergency medicine physician at the University of Utah, performed the first case. Youngquist labeled the catheter as “easy to use and easy to deliver with a smooth insertion.”
Emergency Scientific recently won FDA 510(k) clearance for Landmark with an indication for temporary occlusion of large vessels. That includes patients requiring emergency control of hemorrhage.
CEO Ryan Murri says Landmark offers “another solution for physicians to treat challenging emergency hemorrhage”
“With high quality emergency medicine solutions, we hope to be able to save more patients suffering life threatening injuries,” said Murri. “We believe nobody should die from the loss of blood who could be saved with the right tools, and we look forward to continuing to serve our healthcare partners with additional novel treatment options.”