Elixir Medical today announced it received FDA breakthrough device designation for its DynamX BTK system for treating chronic limb-threatening ischemia. The Milpitas, California-based company designed the adaptive implant to treat narrowed or blocked vessels below the knee (BTK) in patients with chronic limb-threatening ischemia (CLTI).
Related: Elixir Medical wins FDA breakthrough designation for DynamX BTK implant
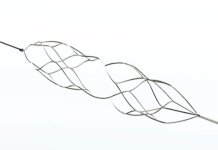
Elixir Medical said the DynamX Bioadaptor metallic device supports the vessel during the healing phase, unlocking and uncaging the vessel while providing essential dynamic support to restore vessel function and maintain an open lumen.
“The Bioadaptor platform was developed to transform treatment of coronary and peripheral artery disease,” CEO Motasim Sirhan said in a news release. “We appreciate the FDA recognition of our innovation for treating the CLTI population with BTK disease and its potential impact on the patients suffering from vascular disease.”
According to the company, the DynamX Bioadaptor has been shown to provide high acute lumen gain in the coronary vessels and maintain such gain over time, which is a common challenge in existing BTK therapies. The bioadaptor has also been shown to restore vessel motion and function, including positive adaptive remodeling, vessel pulsatility, improved vessel dynamic compliance, and an increase in blood flow volume.
The FDA breakthrough device designation accelerates the company’s review process for its technology. Still, through the breakthrough program, it must meet rigorous standards for device safety and effectiveness to be authorized for marketing.